![]()
The Gas Laws
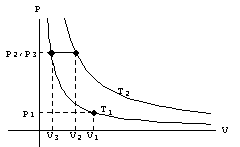
The first “gas law’ was formulated by Robert Boyle in 1662. He related the pressure and volume of a gas in a closed container through his gas law, His experiments were carried out at room temperature, and the law is valid only if the temperature is held constant during the process. We can call the constant C1. Almost a century after Boyle’s work, Jacques Charles and, independently, Louis Gay-Lussac held the pressure of gasses constant and studied the relationship between temperature and volume. Their gas law may be written in the form A graph of Boyle’s law for two different temperatures, and Charles’ law for a particular pressure is shown in the diagram. In the plot of Boyle’s law, T2 is greater than T1. The straight line for Charles’ law is independent of the type of gas (unlike the situation where pressure is plotted as a function of temperature, where the lines for different gasses give different slopes - but all converge at -273.16 oC). This relation also approaches the temperature -273.16 oC as the volume approaches zero.
|
|
|
So, we have two relations involving p,
V, and T (and a third where V is
held constant, if you wish, but two is enough for our purposes). Boyle’s law
can be written as and Charles’ law as ,
the first with T held constant and
the second with p held constant. Look
at the graph of Boyle’s law: A sample of gas starts with pressure p1, temperature T1 and volume V1 and later finds itself at p3, V3 with the temperature held at T1 (i.e. the system parameters move up the T1 = constant curve). We can
then write .
Next our system moves to while holding the pressure constant (Charles’
law). For this case we write Eliminating V3 from these and since we arrive at the more general form of the
combined gas laws as or, equally, Can we find a value for the constant? Remember
that these laws were arrived at experimentally, and further experiments showed
that the constant is directly proportional to the mass (m) of gas used, and inversely proportional to the molecular weight
(M) of the gas. But m is itself directly proportional to M ( where n
is the number of moles of the gas) and so the ratio of these is a constant*. We
thus write (moving the T to the right
hand side, in keeping with tradition),
|
*Say we have a system consisting of a gas of mass m and molecular weight M. The system obeys the gas law, , where C3 is a constant that depends directly on m and inversely on M, as experiment shows. We can just as well write the law as where C4 is still another constant. Now if we double the mass of the gas and at the same time double its molecular weight, the right side of the equation won’t change, and in particular C4 (which we call R) won’t change. Recognizing that is just the number of moles of gas in our system (n), we write
|
This is the ideal gas law, with R the universal gas constant equal to 8.31434. It’s called ideal because it is valid for only so-called ideal gasses. At the time these laws were formulated this meant dilute gasses. As the pressures of the gasses involved were increased, the relation deteriorated. At atmospheric pressures and “ordinary” temperatures, gasses are essentially ideal. What are the criteria for an ideal gas? When treating the gas as a fluid, the answer is “dilute enough”. When treating the gas as a collection of molecules in constant motion the answer becomes much more satisfying, and leads to the kinetic theory of gasses.
|
Here’s an
interesting result of this law: Suppose we take one mole of a gas – any gas –
at “normal” conditions, i.e. room temperature and 1 atmosphere pressure. Then
T = 300 K and p = 1 atmosphere = 1.01 x 105
|