The Quantum Theory
Black Body Radiation and the Ultraviolet Catastrophe
At the turn of the century (19th to
20th), another puzzle was bothering physicists. (It is no wonder that the decade
between 1895 and 1905 is called the golden decade of physics.) A black body was
an object that absorbed every bit of radiation that fell on it and then, as a
consequence of the energy so gained, would reradiate this energy at a frequency
dependent only upon its temperature. If the equations of statistical mechanics
were applied to black body radiation one could expect to determine the intensity
and frequency (of oscillation) of the radiation. Experiments (and common
experience, say with a hot plate, which is a crude example of a black body
radiator) show that the radiation from such a body emits in a wide spectrum of
colors, from deep red to white hot. Maxwell's electromagnetic theory predicts
that the radiation may be of any frequency (even infinitely high), and
statistical mechanics thus predicts that the energy must be equally distributed
among all these frequencies (even an infinite number of them).
| Since the
majority of the energy would thus be among the higher frequencies (i.e.
shorter wavelengths - because you can have many more short wavelengths
than long ones), the emitted light should be dominated by blue or violet
(or ultraviolet) compared to the lower frequency red or orange. Thus
theory predicted what was contrary to observation and brought on what was
called the "ultraviolet catastrophe". The contradiction between
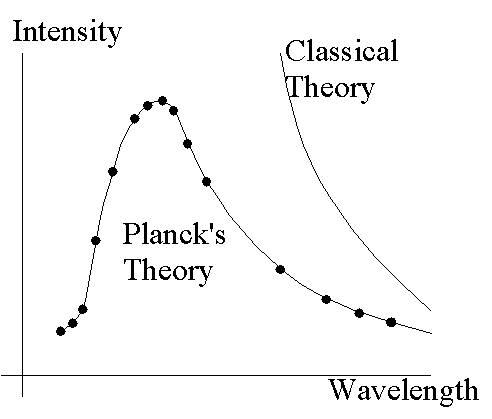
theory and experiment can be seen in the drawing. Electromagnetic theory
and statistical mechanics (i.e. "classical theory") predicted
the rightmost curve; experiment produced the dots. The predictions of Max
Planck (as discussed below) are shown as the curve passing almost
perfectly through the experimental data points.
In classical theory the energy of
a wave is related to its amplitude (big water waves have more energy
than small ones) - the frequency of the wave is not energy related.
In a complete departure Max
Planck postulated a connection between energy and frequency through
his equation E = h f. He sets the energy to be proportional
to the frequency of the radiation, independent of the amplitude. This is
in 1900. In 1905 Einstein, in a paper on the photoelectric effect, uses
Planck's proportionality constant (h) to describe a still different
phenomena. These theories would find common ground with the work of Niels
Bohr, who was trying to understand how Rutherford's model of the atom
could survive in the face of the energy loss it's electrons must suffer
as they orbit the atom's nucleus, as required by classical
electrodynamics. |
 |
Like Planck, Bohr ignored the dictates of
classical theory and instead made three assumptions that would provide for the
needed stability of Rutherford's electron orbits and explain at last the secret
of the spectral lines. He first asserted that electrons circle their parent
atoms in only discrete orbits, and so discrete energies. He tied these orbits to
the angular momentum of the electron through Planck's constant, and assigned to
them "quantum numbers" that defined these "allowed states".
(The specific relation is given by L = n h/2 p,
where the quantum number n can take on the integer values 1, 2, 3, ...,
and h is Planck's constant). Once this was done he solved the problem of
electron energy radiation by postulating that while in one of its allowed
discrete orbits the electrons do not radiate energy. He attempted no proof of
this, he just boldly threw away classical restraints and made an assumption. The
puzzle of the spectral lines he solved by assuming (again without theoretical
foundation) that the electrons could gain energy from outside stimulation if
this energy exactly moved the electrons between allowed orbits. Once in one of
these higher energy states the electrons would spontaneously drop to a lower
energy state, there by giving back their energy (i.e. radiating it). As this
energy was well defined it would, by Planck's equation (E
= h f), emit its energy at a well
defined frequency and so as a distinctive color, as observed in spectroscopy.
Bohr presented his revision to
Rutherford's atom in 1913. From then
until the late 1920's the new physics was treated to extreme scrutiny. Names
such as Schrodinger, De Broglie, Pauli, Dirac and many others became famous as
they expanded the theory. Einstein, who published his work on general relativity
in 1916, was a major contributor to quantum theory. I must stop here in the
story. As a footnote, I can say that the quantum theory (quantum mechanics) has
developed to a point where its foundations are very strong. It has met every
test without failure. It has encompassed relativity and, in addition to solving
puzzles on the atomic scale, has given us the tools to open our knowledge of the
universe on the largest scale, and helps provide answers to questions of the
origin of the universe itself.
Go on to the origin
and structure of the universe.
