Atomic Spectra
In 1666 Newton allowed a thin beam of
sunlight to fall upon a prism. The light spread into a rainbow of colors
providing a "pleasing divertissement to view the vivid and intense colors
produced thereby". He then recombined the colored rays using another prism
and again got back the original white light. Clearly, white light was really a
mixture of all colors combined. By combining a telescope with a prism,
Fraunhofer, in 1814, was able to spread sunlight out enough to observe
dark lines mixed within the rainbow of colors, and began the study of
spectroscopy. Further work by Bunsen and Kirchoff in 1859 explained the dark
lines. They noted that when a flame containing sodium was observed with a
spectrometer, two bright yellow lines (among others) was always present. These
lines, previously noticed by Fraunhofer in a candle flame, were the identifying
mark of sodium. From this came the fundamental laws of spectroscopy: Each
element has a unique set of spectral lines, and these can exist as bright
colored lines from a source emitting light (e.g. a gas excited by an electrical
discharge, as in a neon sign), or as dark lines against a continuum of colors
(e.g. when white light is shown through a cool vapor of neon).
It was time when Newton's laws had
solidified mechanics and Maxwell's electromagnetic theory had united the fields
of electricity, magnetism and light. There was, in the minds of some physicists,
left little remaining to do in their field. The matter of understanding the
fundamental principles behind the spectral patterns arising from the elements
was the first step that led to a new and totally unexpected physics.
The Electron
As the 19th century was coming to a close, three new phenomena
had caught the attention of physicists. Rontgen's X-rays, created when high
voltages were imposed on a vacuum tube, Becquerel's radioactivity from which new
rays were emitted from uranium and the newly identified (by Marie Curie) element
radium, and the electrical discharge in rarified atmospheres that eventually led
to the discovery of the electron by J.
J. Thompson. The radioactive rays were named alpha rays, beta rays and gamma
rays. Gamma rays were quickly associated with the X-rays of Rontgen, but the
other two had not been observed elsewhere.
Thompson's experiments were built on the popular but
unexplained experiments using "Crook's tubes": A glass tube has the
air pumped from it, and a high voltage connected between electrodes at either
ends of the tube. As the voltage is increased the tube glows brightly. If a mask
is inserted in the tube its shadow appears on one end, a clear indicator that a
beam of particles is being projected from one end of the tube (the negative, or
cathode end) to the other (positive, or anode) end. Because these "cathode
rays" carried an electric charge, Thompson was able to deflect with
electric and magnetic fields. From this he conjectured that these cathode rays
were in fact particles of matter that were "atoms, or molecules, or matter
in a still finer state of subdivision?", and was able to determine their
ratio of charge to mass. In a later elegant experiment Robert
Millikan balanced the downward gravitational force on tiny charged droplets
of oil by an upward electrical force and thereby measured the electrical charge.
Once this was done the mass was immediately known and the beginnings of a
quantitative assessment of the structure of the atom was under way.
Thompson's Atom - and Rutherford's Correction
If each atom had its own signature in the
form of spectral lines, what was the source of these lines? Thompson thought he
had the answer. Maxwell had shown that an accelerating charge radiated energy.
Of cathode rays Thompson wrote "I can see no escape from the conclusion
that they are charges of negative electricity carried by particles of
matter." These rays (now particles in Thompson's view, later named
electrons) when made to oscillate could account for the atomic spectra.
| Building
on a model proposed by Kelvin in 1902, Thompson's model of the atom
consisted of a region of positive charge within which were embedded the
negatively charged electrons. The positive distribution of charge just
balanced the charge of the electrons as was required to assure overall neutrality
of the atom. When the atom was excited (by a flame, perhaps) the resulting
oscillations gave rise the spectra. If the excitation were great enough
(as in the case of Crook's tube experiments) cathode rays would result.
Many problems arose when this model was put to the test, but it was a
significant beginning.
Thompson's atom was constructed
using the laws of Newton and Maxwell, and there where questions
unanswered: His model used the mutually repelling force of the electrons
within the positive cloud to establish stable atoms, but such a model was
hard pressed to explain the necessary radiation patterns needed to match
his theory with the observed spectral lines. A theory must be
mathematically as well as conceptually plausible. Thompson's work was done
while he was director of the Cavendish Laboratory at Cambridge University
where, in 1890 he was joined by Ernest
Rutherford, a young physics student from New Zealand.
|
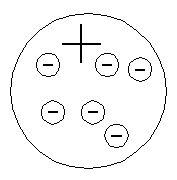
Thompson's Atom |
| Rutherford began to
investigate the recently discovered properties of radioactivity. One
characteristic of the radioactive materials discovered by Henri Becquerel
was that when the emissions from a radioactive source were formed into a
beam, and the beam allowed to pass through electric and/or magnetic
fields, the beam would be separated into three distinct portions labeled
alpha, beta and gamma. The deflections resulting from the fields
determined that the alpha beams consisted of massive, positively charged
particles (later determined to be Helium nuclei), the beta beams were
light, negatively charged particles (Thompson's electrons) and the gamma
rays were uncharged (and so difficult to characterize, but eventually were
determined to be high energy electromagnetic radiation). A diagram of the
apparatus is shown to the right. |
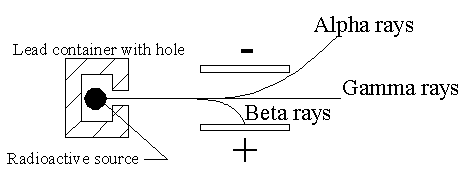 |
| The alpha particles
carried a charge twice that of the electron and were about 8,000 times
heaver. Because of their mass and charge they were used as projectiles to
bombard thin targets and so react with the atoms of the target material.
Rutherford directed a beam of alpha particles at a thin film of gold, in
an attempt to explore the character of Thompson's atomic model. one would
expect that the atoms of the foil would deflect the alpha particles over a
range of angles as they passed through the positive boy of the atoms.
Instead, most of the particles went through with only small deflections,
but an occasional alpha particle would be deflected almost directly back
towards the source. This was completely foreign to what was predicted by
the Thompson model, which caused Rutherford to write "It was almost
as incredible as if you fired a 15-inch shell at a piece of tissue paper
and it came back and hit you." To explain the effect he proposed a
different model for the atom in which the positive charge was concentrated
in a tiny region at the center, and the electrons orbited about, much as
planets orbit the Sun. This model, familiar to all school children, is
shown here. |
 |
While Rutherford's model was successful
in explaining his scattering experiment, it had a fateful flaw: Unlike planets
orbiting our Sun, the electrons orbiting Rutherford's nucleus carried an
electrical charge. As these charges go around, they accelerate and so, according
the electromagnetic theory, must radiate energy. This process is paramount in,
for example, the transmission of radio and television signals. This flaw was to
be removed only at the cost of the sanctity of the laws of Newton and Maxwell.
Go to the page on
quantum theory


